Background: Ibrutinib has dramatically changed the treatment landscape for relapsed/refractory (R/R) and newly diagnosed CLL/SLL patients (pts), leading to durable remissions in the majority of pts; however, this comes at a high cost to the healthcare system particularly since treatment is continued until progression. Ibrutinib has been available in BC on compassionate access basis since 2014, publicly funded for R/R CLL/SLL or upfront for pts with deletion 17p (del17p) since 2016, and as upfront therapy in fludarabine-ineligible pts since 2018. In view of Ibrutinib's widespread use and rapid uptake among BC physicians, we sought to investigate discontinuation rates, dose modifications, and overall survival (OS) with introduction of ibrutinib within the publicly funded BC healthcare system.
Methods: CLL/SLL pts ≥ 18 years (y) who initiated single-agent ibrutinib in routine practice in BC from Nov. 2014-Jun. 2018, with minimum of 6 months follow-up (f/u), were included. Pts were identified retrospectively using provincial databases. Patient level information on dates of ibrutinib and dose modifications were obtained from the BC Cancer Pharmacy Database.
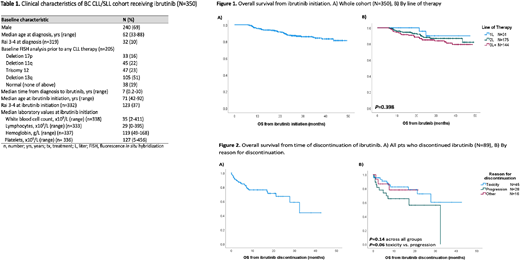
Results: 350 CLL/SLL pts were identified who received ibrutinib for first line (1L, n=31) or R/R disease (2L, n=175; 3L+, n=144) at a median 7.3 y (range, 0.17 - 30) from CLL diagnosis. Most pts were male (69%) with Rai stage 0-II (287/319, 90%) at CLL diagnosis. FISH abnormalities prior to any therapy, among 205 pts tested, were: 16% del17p, 22% del11q, 23% trisomy 12, 51% del13q and 19% no abnormalities.
Median age at ibrutinib treatment was 71 y (range, 42-92) and pts with R/R disease had a median of 2 prior treatment lines (range, 1-13). At ibrutinib initiation, most pts had low-intermediate Rai stage (209/332, 63%). Median f/u from ibrutinib initiation for living pts was 27.2 months (mos) (range, 6.1-49.4). Median starting dose was 420 mg daily (range, 140-560) and median duration of ibrutinib was 22.3 mos (range, 0-49.4), with 261 pts (75%) alive and remaining on ibrutinib at last f/u. Dose reductions occurred in 32% (104/322) of pts, most commonly for toxicity (86/104, 83%). Other reasons for dose reductions included new comorbidities, n=2; medication interactions, n=3; incorrect starting dose, n=1; other causes, n=10; and unknown, n=2. Temporary dose interruptions recorded by physicians were observed in 114/305 pts (37%). A total of 89 pts (25%) discontinued ibrutinib for reasons other than death: toxicity, including infections and/or cardiac events, n=45 (51%); progression, n=28 (31%); and other reasons, n=16 (18%) (6 patient/physician choice, 4 SCT, 4 new comorbidity/drug interaction, 2 change in goals of care). Pts who discontinued treatment for toxicity and progression had median exposure times of 10.4 mos (range, 0.8-31.4) and 11.9 mos (range, 1.0-32.8), respectively. An additional 21 pts (6%) died while on ibrutinib: 9 from CLL progression; 5 cardiac causes (2 ischemic heart disease; 1 myocardial infarction; 1 cardiac arrest; 1 ventricular tachycardia); 4 infection; and secondary malignancy, COPD and unknown in 1 pt each.
From time of ibrutinib initiation, 2 y OS for the whole cohort was 87.4% (95% CI: 83-91%) with median not reached (Fig. 1A). By line of therapy, 2 y OS from ibrutinib was 95.7% for 1L, 88.0% 2L, and 84.9% 3L+, with no significant difference between groups (P=.4) (Fig. 1B). Pts who discontinued ibrutinib, excluding those who died on ibrutinib, had a median OS from time of discontinuation of 32.5 mos (95% CI: 23.4-41.6) (Fig. 2A), with no significant difference based on reason for discontinuation (2 y OS from discontinuation for toxicity, progression or other was 72.4 %, 56.1%, and 78%, respectively, P=.14; P=.06 for toxicity vs progression) (Fig. 2B).
Conclusion: This real-world analysis of CLL/SLL pts on ibrutinib confirms excellent survival in both the front-line and R/R settings; however, after 2 years on ibrutinib therapy, 25% of pts discontinued treatment and had poor outcomes regardless of the reason for discontinuation. This data serves as a benchmark to assess healthcare utilization with the introduction of ibrutinib at a population-level, in order to assess its cost-effectiveness and sustainability.
Savage:Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie: Honoraria; Roche (institutional): Research Funding; BeiGene: Other: Steering Committee; Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie, Servier: Consultancy. Villa:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; AZ: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria; Nano String: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Sandoz Canada: Consultancy, Honoraria; Immunovaccine: Consultancy, Honoraria; Purdue Pharma: Consultancy, Honoraria. Scott:NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoString, Research Funding; Celgene: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy, Research Funding; NIH: Consultancy, Other: Co-inventor on a patent related to the MCL35 assay filed at the National Institutes of Health, United States of America.; Roche/Genentech: Research Funding. Sehn:Chugai: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Teva: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Genentech, Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Verastem Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria. Toze:Janssen: Consultancy, Other: advisory board. Gerrie:Astrazeneca: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Sandoz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal